Therapeutic
peptides
Peptides used for therapeutic purposes are original active ingredients and a blend of chemical entities and proteins. They display the characteristics of both the former as they are usually chemically synthesised, and those of the latter as their primary structure is made up of amino acids and peptide bonds.
The active ingredients found in peptides are generally strong, but they have the distinct disadvantage of not being orally bioavailable and are often required for long-term treatment. This makes peptides ideal candidates for a sustained-release injectable delivery (injectable depot formulations).
The development of a peptide-based medicine is quite an art, as the chemical development strategy must be well articulated with the regulatory pre-clinical strategy and the formulation strategy.

Aptys pharma® possesses this experience, namely through its CEO, François Boutignon who filled the post of vice-president responsible for R & D at Europeptides, a biotech company established in 1991, then at the French subsidiary of Mediolanum Farmaceutici, before moving on to Asta Medica. During this time, François developed several innovative peptide-based products, including injectable depot formulations of GnRH analogues (biodegradable implants, microspheres, and microcrystals).
Aptys pharma® has, since its inception, been regularly tasked to design peptide-based medicines and to provide its clients with not just strategic advice, but also to develop and validate suitable quality control methods for evaluating these formulations. The company has notably developed injectable biodegradable implants of GnRH agonists and injectable biodegradable microspheres of GnRH antagonists.

Soluprec®
Preclinical formulation, with its many constraints, can be a real challenge. Preclinical studies (pharmacology and toxicity) require increasing doses of active substances, while the volume for administration in animals is limited.
Aptys Pharma has developed SOLUPREC®: a simple, rapid approach to meeting this challenge, particularly for poorly soluble molecules, which account for more than 70% of new chemical entities.
Thanks to our integrated approach, we can significantly reduce the time needed to move from discovery to preclinical development, while ensuring strict compliance with regulatory standards. With SOLUPREC®, we optimize the use of the active ingredient, reducing the amount needed for testing.

SoluDiag®
Between 70% of new research-driven chemical molecules suffer from problems of solubility resulting often in poor bioavailability. In addition, in the earliest stages of development, pharmaceutical and biotechnology companies rarely have large quantities of material at their disposal. And finally, these companies have a defined and urgent need to launch regulatory preclinical toxicology and non-regulatory pharmacology studies to validate molecule development using rapid, reliable, and replicable molecule-testing methods.
In reply to these needs, Aptys pharma® developed SoluDiag®, a progressive and rapid method of testing active ingredient solubility that uses little raw material.
SoluDiag® is a three-stage approach called E.S.C., as in:
- Exploring candidate molecule solubility, whereby between 20 and 30 solvent and co-solvent conditions are tested.
- Selecting the most satisfying conditions which are then checked and confirmed by spectrophotometer transmittance analysis.
- Confirming the solubility of the best condition by HPLC.
Just 100mg of active ingredient are required. The solvents and co-solvents are chosen as per their acceptability in vitro and in vivo on animals, or with a view to clinical studies on humans.
SoluDiag® technology makes it possible to define a liquid (solution) or semi-solid (suspension or emulsion) formulation strategy for a low solubility active ingredient.


NanoSolution®
There are several techniques to improve the bioavailability of poorly soluble active ingredients, these currently are:
enhancing solubility by using solvents, co-solvents, cyclodextrins, or by changing salts, using solid solutions, using the extrusion-spheronisation process, and finally, nanoscale grinding to shrink the particle size using ball milling to increase the specific surface of insoluble particles, and as a result the speed of dissolution and bioavailability.
Yet, one problem with ball milling is that it requires a lot of raw material and is time consuming as every composition must be tested to get the perfect particle size.
To overcome this problem, Aptys pharma® in cooperation with Losan Pharma, has developed NanoSolution®, a quick way to carry out experimental designs using little raw material.
NanoSolution® is based on a dual-rotation centrifugal ball milling technique using a Zentrimix 380R (Hettich) dual-rotation centrifuge that can test up to forty different conditions (ball size, polymers, surfactants etc.) in less than two hours.
A Mastersizer 2000 (Malvern) laser diffraction analyser determines the particle size and distribution in nano suspensions.
Nano-suspensions are then dissolution tested in vitro using a SOTAX AT7 or USP4 calibrator or in vivo using rats. Then formulated by freeze-drying or using a fluidised air bed prior to compression and capsule filling.
The quantities of raw material needed for the initial tests can be in the order of just a few grams.

Freeze drying
Nano solutions developed using the NanoSolution® technique can be freeze dried in a first formulation stage with a Christ Epsilon 2-6D freeze dryer. Fitted with three shelves, the machine’s capacity can be adapted from a minimum 189 vials (20R) to 702 vials (4R and 2R). It is equipped with an arm that allows the extraction of vials for analysis during the lyophilisation cycle. Its ice condenser capacity is 8kg at -75°C.
This equipment is perfect for the freeze-drying of peptides and proteins and for small batches for testing on animals or for stability testing.

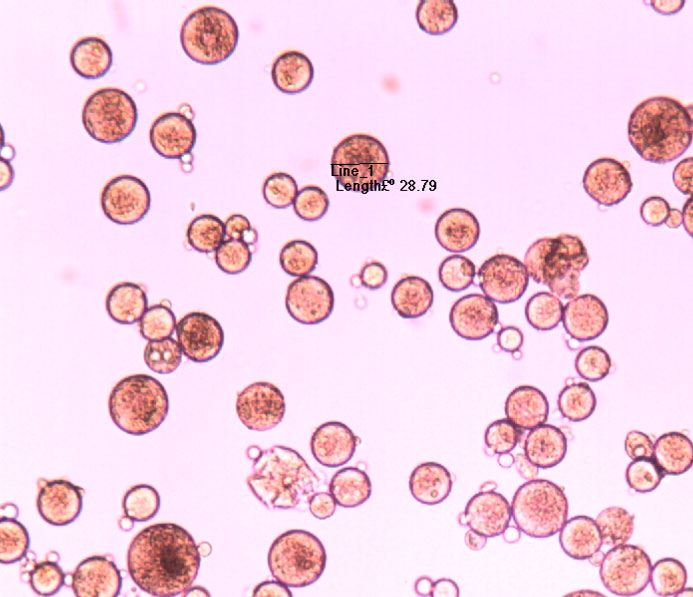
Microspheres
Aptys pharma® is in a position to design injectable, controlled-release microspheres for the delivery of therapeutic peptides for experimenting on animals. These microspheres are designed by conventional coacervation or double emulsion techniques using biodegradable polyesters such as PLA or PLGA prior to calibration using a SOTAX AT7 or USP4 calibrator or in vivo using rats. The firm has also developed injectable biodegradable microspheres of GnRH antagonists.

Coating &
Granulation
Using our ProCepT 4M8-Trix fluid bed dryer, we are able, at Aptys pharma®, to dry, granulate and coat powders, granules, and tablets in both aqueous and organic phases. The advantage of this apparatus is that it only uses small quantity batches from 50 to 200 grams in “top spray” or “bottom spray” modes. It can be used for formulation screening, taste masking, gastric and enteric protection coating, and coating for sustained release formulations. The resulting powders can then be formulated into capsules or tablets. Aptys pharma® has notably developed formulations of delayed morphine delivery.



Tablets
Aptys pharma® has extensive experience in the design of tablets and has developed numerous formulations of both immediate and delayed delivery formats. For example, Loramyc®, the mucoadhesive buccal tablet, first licensed to and marketed by Bioalliance Pharma in 2005, prior to Vectans Pharma, was originally developed by Aptys pharma®. And more recently, Aptys pharma® in partnership with Unither has developed Metapain® a combination of nefopam and paracetamol.
Using Erweka granulators and Turbula blender mixers harnessed to a Forgerais MR6 6-station rotary tablet press, Aptys pharma® is able to develop and produce tablets of differing shapes and sizes for stability testing batches, for in vitro or animal testing. Aptys pharma® also owns a unique machine for producing micro-tablets for experimental purposes on animals. Using very small quantities, this apparatus is capable of producing tablets with a 1.5mm diameter.
The company has all the necessary equipment to evaluate tablet characteristics, such as a Sotax hardness tester, a Precisa Halogen precision laboratory scales, Sotax friability tester, Sotax tamping machine and a Sotax disintegration tester.

Liquid and semi-solid formulations
Aptys pharma® also has extensive experience in the design and development of liquids and semi-solid formulations having designed many transdermal and vaginal formulations using different hormone types based on the BiGel technology that was acquired in 2002. Testocream®, a transdermal testosterone gel-cream for treating male hypogonadism, marketed by Viramal Ltd, was first developed by Aptys pharma®.
Aptys pharma® is equipped with a complete array of equipment enabling it to design formulations weighing just a few hundred grams to 10kg, these include an IKA Ultra Turrax disperser, a VMI Turbotest mixer, a VMI/Rayneri Trimix homogeniser mixer and to analyse them a Realux polarising light microscope and a Lamy viscosity meter.